How Do Chemists Organize Information About Elements
Scientists then came up with the idea that they could arrange the element by the atomic mass number. Be able to tell.
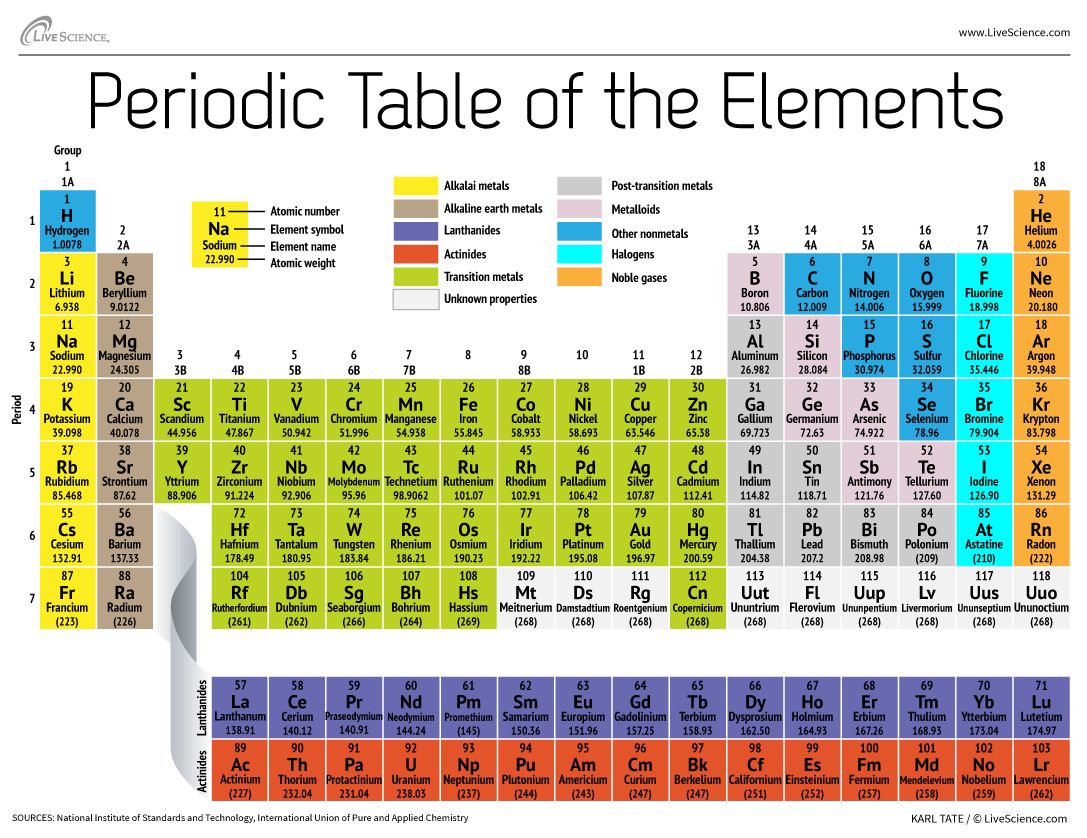
How The Periodic Table Groups The Elements Live Science
Mendeleev arranged the elements in his periodic table in order of increasing atomic mass.

. Every atom of hydrogen has 1 proton. Chemists used the properties of elements to sort them into groups. Elements that have similar chemical and physical properties end up in the same column in the periodic table.
How do chemists organize information about elements. The periodic table is an icon. How did chemists organize the elements that were known.
There were almost 120 known elements as of 2012. Chemists organize information about atoms. Start with Set A.
Chemistry published 12052018 When two forces act in opposite directions the object will accelerate in the same direction as the blank force. Being organized often means putting items in an order that makes them easier to use Figure2 The same thing goes for the elements that make up all of matter. Every element has its own unique atomic mass number because elements have different numbers of protons and neutrons.
Until a new element is discovered the last element on the table is element number 118. 51203 g of water at 552 degree c is added to a calorimeter containing 49783 g of water at 235 c. The table lists the chemical elements in order of increasing atomic number which is the number of protons in an atom of an element.
In the modern periodic table elements are arranged in order of increasing atomic number. In their atoms the s and p sublevels in the highest occupied. In short the periodic table is more than just a table.
Elements are pure substances that cant be separated into other substances. Most non-metals were gases and liquids brittle and poor conductors of heat and electricity. Thats a lot of elements to remember.
An element in an A group in the periodic table. Chemistry published 12052018. Chemists and materials scientists typically do the following.
Chemical elements are organized in the periodic table of Mendeleev. Every atom of element 118 has 118 protons. After stirring and waiting for the system to equilibrate the final.
Through development of quantum mechanics. Scientists then came up with the idea that they could arrange the element by the atomic mass number. Acalorimeter is to be calibrated.
An element in an A group in the periodic table. As a group these elements display a wide range of physical and chemical properties. 1 Show answers Another question on Chemistry.
In the modern periodic table elements are arranged in order of increasing atomic number name the three broad classes of elements. This is the biggest difference between todays periodic table and Mendeleevs periodic table. When elements are arranged in order of increasing atomic number there is a periodic repetition of their physical and chemical properties.
The original table organized the elements by increasing atomic weight. The arrangement of the elements into periods has an important consequence.

Standard Atomic Weights Of 14 Chemical Elements Revised Iupac International Union Of Pure And Applied Chemistry Chemistry Chemical Atom

Matter Chemical Change Ppt Download

The Hidden Structure Of The Periodic System Data Visualization Design Chemical Bond Covalent Bonding

Elements And The Periodic Table Introductory Chemistry Lecture Lab
No comments for "How Do Chemists Organize Information About Elements"
Post a Comment